Borane-catalysed postpolymerisation modification of the Si–H bonds in poly(phenylsilane)†
Abstract
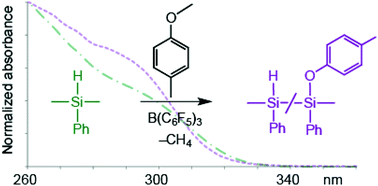
B(C6F5)3-catalysed hydrosilation, heterodehydrocoupling, and demethanative coupling reactions of the Si–H bonds in poly(phenylsilane) allow the introduction of 10–40% new sidechains in this polymer. The resulting new polymers contain an unusually wide variety of functionalities including Si–C, Si–O, Si–N, and Si–S bonds, whose presence is confirmed by NMR and IR spectroscopies. Gel permeation chromatography (GPC) and thermogravimetric analysis (TGA) are consistent with conservation of the all-silicon backbones in these modified polymers, a result of the high chemoselectivity of the borane-catalysed reactions for Si–H versus Si–Si bonds. UV-visible spectroscopy is sensitive to the presence of new functional groups in the modified polysilanes, although the high proportion of residual Si–H groups attenuates the changes in the σ-delocalized chromophores. Limitations in substrate scope, arising from issues of borane-substrate complexation or competing catalytic over-reduction chemistry, have been identified, and the potential for achieving greater degrees of sidechain substitution at higher reaction temperatures has been demonstrated for the hydrosilation of 1-hexene.
- This article is part of the themed collection: Silicon Chemistry: Discoveries and Advances


 Please wait while we load your content...
Please wait while we load your content...