Application of the boron center for the design of a covalently bonded closely spaced triad of porphyrin-fullerene mediated by dipyrromethane†
Abstract
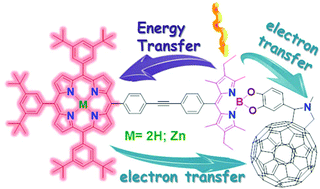
Two novel triads had been designed through covalent bond connection of the boron dipyrromethane (BODIPY), free base porphyrin (H2P) or zinc(II) porphyrin (ZnP) and N-methyl-2-phenyl-3,4-fulleropyrrolidine (C60) mediated by BODIPY. This closely spaced triad arrangement where porphyrin and fullerene are placed apart is anticipated to stabilize charge separation by separating the two radicals from each other. Two model polyads were synthesized with BODIPY and H2P or ZnP to investigate interaction between the two chromophores. Photo-excitation of the BODIPY triggered an efficient singlet energy transfer where the rates are found to be ∼1010–1011 s−1. For triads with C60 fast electron transfer was confirmed by the detection of the C60˙− signature from femtosecond transient absorption (fs-TA) in ∼0.4–3 ps. The charge recombination is estimated to be in the nanosecond window. This indicates the convenience of this arrangement for stabilizing the charge-separated state.
- This article is part of the themed collection: CSC100: Celebrating Canadian Chemistry


 Please wait while we load your content...
Please wait while we load your content...