Isocyanide insertion across the Pd–C bond of allenyl and propargyl palladium complexes bearing phosphoquinoline as a spectator ligand. Synthesis of a palladium complex bearing a coordinated cyclobutenyl fragment†
Abstract
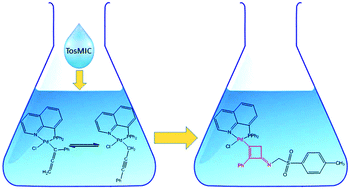
We have studied the insertion of p-toluenesulfonylmethyl isocyanide (TosMIC) on selected allenyl and propargyl complexes of palladium bearing diphenylphosphine quinoline as a spectator ligand. The fast process gives different products depending on the tautomer involved in the reaction. Thus, the unsubstituted allenyl species yields an insertion complex with the isocyanide coordinated between the metal and the first allenyl carbon. On the other hand, a mixture of phenyl substituted allenyl and propargyl palladium complexes yields a novel species characterized by a cyclo-butenyl fragment directly coordinated to palladium. The solid state structure of such a complex together with an exhaustive kinetic study of the whole process is reported.


 Please wait while we load your content...
Please wait while we load your content...