Metal-free pincer ligand chemistry polycationic phosphonium Lewis acids†
Abstract
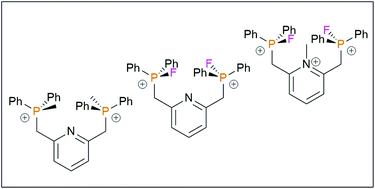
Oxidation of 2,6-bis(diphenylphosphine)methyl pyridine with MeO3SCF3, yields the dication [2,6-(CH2PMePh2)2C5H3N][CF3SO3]22, while subsequent treatment with two equivalents of [K(THF)n][B(C6F5)4] (n = 1.3–1.5) afforded [2,6-(CH2PMePh2)2C5H3N][B(C6F5)4]23. Analogous treatment using XeF2 afforded 2,6-(CH2PF2Ph2)2C5H3N 4, while subsequent fluoride abstractions afford the corresponding [BF4], [CF3SO3] and [B(C6F5)4] salts, 5–7, respectively. Compound 4 reacts with an excess of MeO3SCF3 to give [2,6-(CH2PF2Ph2)2C5H3N(CH3)][CF3SO3] 8 while subsequent reaction with [Et3Si·2C7H8][B(C6F5)4] gave the related tricationic species, 9. The Lewis acidity of these di- and triphosphonium cations 3, 7 and 9 was examined and their utility as Lewis acid catalysts was assessed in the dimerization of 1,1-diphenylethylene, the hydrodefluorination of 1-fluoroadamantane, and the dehydrocoupling of phenol and silane.


 Please wait while we load your content...
Please wait while we load your content...