Proton conduction in a hydrogen-bonded complex of copper(ii)-bipyridine glycoluril nitrate†
Abstract
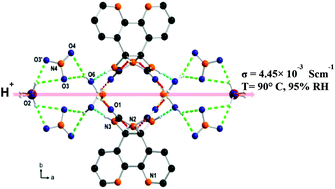
Bipyridine glycoluril (BPG), a urea-fused bipyridine tecton, forms a square-pyramidal secondary building unit with copper(II) which further self-assembles to give a porous hydrogen-bonded complex. This complex displays a high proton conductivity of 4.45 × 10−3 S cm−1 at 90 °C and 95% relative humidity (RH). Chains consisting of coordinated water, solvent water and nitrate anions embedded in the complex are responsible for high proton conduction. The proton conduction pathway was corroborated by ab initio electronic structure calculations with molecular dynamics (MD) simulations using the Nudged Elastic Band (NEB) method. The theoretical activation energy estimated to be 0.18 eV is in close agreement with the experimental value of 0.15 eV which evidences a Grotthuss proton hopping mechanism. We thus demonstrate that the hydrogen-bonded complex encapsulating appropriate counter ions, coordinated water and solvent water molecules exhibts superprotonic conductivity.


 Please wait while we load your content...
Please wait while we load your content...