Boric acid-destabilized lithium borohydride with a 5.6 wt% dehydrogenation capacity at moderate temperatures†
Abstract
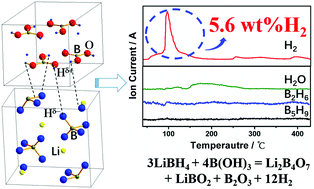
Boric acid effectively promotes the dehydrogenation of lithium borohydride due to the interactions between protonic and hydridic hydrogen. The simple mixture of LiBH4–(4/3)B(OH)3 can release 5.6 wt% hydrogen below 180 °C with only a trace amount of water, thus constituting a highly attractive single-use hydrogen storage material.


 Please wait while we load your content...
Please wait while we load your content...