Abstract
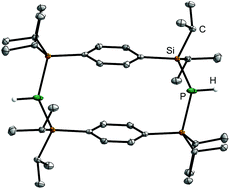
Heteroatomic bridged paracyclophanes were obtained by two independent synthetic approaches. The required precursors consist of para R2SiCl (R = Me, iPr) substituted aromatic rings (2 and 4). They were subsequently functionalised by using NH3, [LiPH2(dme)] or LiAl(PH2)4. In the case of the Me-substituted species 2, the reaction with NH3 directly yielded the Si2N bridged paracyclophane 5. The Si2P incorporated derivative 10 was obtained by lithiation of p-C6H4(SiiPr2PH2)2 (9) and subsequent salt metathesis with the chlorosilane 4. The second approach involves the use of GaEt3 in the formation of four membered (GaPn)2 cycles (Pn = N, P). p-[C6H4{SiiPr2N(H)GaEt2}2]2 (11) and p-[C6H4{SiiPr2P(H)GaEt2}2]2 (12) represent the first examples of stable (GaPn)2cis isomers as the trans species did not appear in solution. Although 11 and 12 show a similar coordination pattern, they differ in the orientation of the aromatic systems: in the solid structure, 11 adopts a – for paracyclophanes so far unique – T-shape conformation of the phenyl rings, while 12 shows the predominant coplanar orientation. All cyclophanes were characterized by X-ray diffraction, elemental analysis, NMR and IR spectroscopy.


 Please wait while we load your content...
Please wait while we load your content...