Cationic rhenium(iii) complexes: synthesis, characterization, and reactivity for hydrosilylation of aldehydes†
Abstract
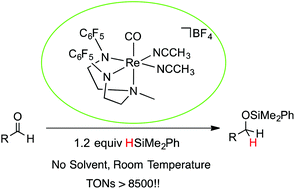
A series of novel cationic Re(III) complexes [(DAAm)Re(CO)(NCCH3)2][X] [DAAm = N,N-bis(2-arylaminoethyl)methylamine; aryl = C6F5 (a), Mes (b)] [X = OTf (2), BArF4 [BArF4 = tetrakis[3,5-(trifluoromethyl)phenyl]borate] (3), BF4 (4), PF6 (5)], and their analogue [(DAmA)Re(CO)(Cl)2] [DAmA = N,N-bis(2-arylamineethyl)methylamino; aryl = C6F5] (6) were synthesized. The catalytic efficiency for the hydrosilylation reaction of aldehydes using 4a (0.03 mol%) has been demonstrated to be significantly more active than rhenium catalysts previously reported in the literature. The data suggest that electron-withdrawing substituents at the diamido amine ligand increase the catalytic efficiency of the complexes. Excellent yields were achieved at ambient temperature under neat conditions using dimethylphenylsilane. The reaction affords TONs of up to 9200 and a TOF of up to 126 h−1. Kinetic and mechanistic studies were performed, and the data suggest that the reaction is via a non-hydride ionic hydrosilylation mechanism.


 Please wait while we load your content...
Please wait while we load your content...