LiMCO3 (M = K, Rb, Cs): a series of mixed alkali carbonates with large birefringence†
Abstract
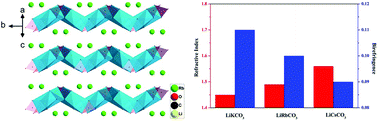
By the hydrothermal method, two new mixed alkali carbonates, LiRbCO3 and LiCsCO3 were synthesized for the first time. Both compounds crystallize in the monoclinic space group P21/n, and exhibit similar wave like [LiCO3]∞ layers connected by Rb–O and Cs–O bonds, respectively. They not only possess low melt temperature and wide spectral transmittance range, but also have relatively large birefringence confirmed by first principles calculations. In the mixed alkali carbonates system of LiMCO3 (M = K, Rb, Cs), with the increase of atomic radius of the alkali metal, the birefringence is reduced from 0.11 in LiKCO3 to 0.09 in LiCsCO3, while the refractivity is increasing. The increasing refractivity and decreasing birefringence can be interpreted by the enhancement of orbital hybridization resulting from the reduced C–O bond length and weakened structural anisotropy, respectively.


 Please wait while we load your content...
Please wait while we load your content...