ACaBO3 (A = Cs, Rb): two new cubic borates with isolated BO3 groups†
Abstract
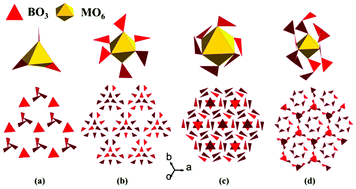
Two alkali metal and alkaline-earth metal borates, ACaBO3 (A = Cs, Rb), were synthesized by a high temperature solution method. The powder SHG response of CsCaBO3 is approximately one time that of KH2PO4 (KDP), and its experimental band gap is 4.20 eV. The SHG density calculations indicate that the SHG effect originates from the synergistic effect of B–O and Ca–O groups. The two compounds crystallize in the cubic space group P213 and their structures feature a 3D framework composed of the CaO6 octahedra, the CsO9 polyhedra and the BO3 triangles. However, borates containing anisotropic planar BO3 triangles exhibit difficulty in forming an isotropic cubic structure. Through our investigation on all the ordered cubic structures with the BO3 or the B2O5 (composed of two BO3) groups, the MO6 octahedra play important role in their structure formations. This discovery is essential for the design of new cubic borates containing the BO3 triangles.


 Please wait while we load your content...
Please wait while we load your content...