Highly selective synthesis of substituted (E)-alkenylsilatranes via catalytic trans-silylation and mechanistic implications†
Abstract
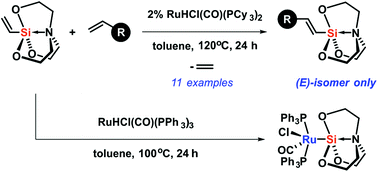
A new route for the synthesis of functionalized alkenylsilatranes has been developed based on ruthenium-catalyzed trans-silylation with olefins. This transformation allowed for the synthesis of new (E)-alkenylsilatranes in good yields and excellent selectivity. Experimental studies concerning the reaction mechanism were carried out and the intermediate ruthenium–silatranyl complex was isolated and characterized. Moreover, detailed DFT calculations regarding the mechanism of the silylative coupling catalytic cycle of silatranes catalyzed by [Ru]–H complexes were also performed.


 Please wait while we load your content...
Please wait while we load your content...