Generalization of the Tolman electronic parameter: the metal–ligand electronic parameter and the intrinsic strength of the metal–ligand bond
Abstract
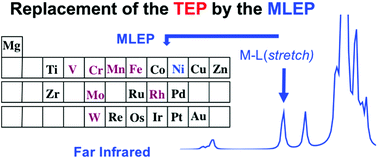
The catalytic activity of transition metal complexes (R)nM–L can be predicted utilizing the metal–ligand electronic parameter (MLEP) that is based on the local stretching force constant of the M-L bond. Vibrational spectroscopy is an excellent tool to accurately determine vibrational mode properties such as stretching frequencies. These correspond, because of mode–mode coupling, to delocalized vibrational modes, which have to be first converted into local vibrational modes, and their properties. Each bond of a molecule can be uniquely characterized by the local stretching force constant and frequency. The former is ideally suited to set up a scale of bond strength orders, which identifies weak M–L bonds with promising catalytic activity. It is shown how the MLEP replaces the TEP (Tolman Electronic Parameter), which is based on the CO stretching frequencies of a (CO)nM–L complex and which is now exclusively used in hundreds of investigations. However, the TEP is at best a qualitative parameter that suffers from relatively large mode–mode coupling errors and the basic deficiency of most indirect descriptors: They cannot correctly describe the intrinsic M–L bond strength via the CO stretching frequencies.
- This article is part of the themed collection: 2017 Frontier and Perspective articles


 Please wait while we load your content...
Please wait while we load your content...