A dinuclear biomimetic Cu complex derived from l-histidine: synthesis and stereoselective oxidations†
Abstract
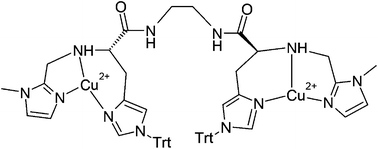
A dinuclear copper(II) complex derived from the chiral N6 ligand (2S,2′S)-N,N′-(ethane-1,2-diyl)bis(2-((1-methyl-1H-imidazol-4-ylmethyl)-amino)-3-(1-trityl-1H-imidazol-4-yl)propanamide) (EHI) was synthesized and studied as a catalyst in stereoselective oxidation reactions. The ligand contains two sets of tridentate binding units, each of them giving rise to a coordination set consisting of a pair of 5- and 6-membered chelate rings, connected by an ethanediamide linker. Stereoselectivity effects were studied in the oxidations of a series of chiral L/D biogenic catechols and the pair of L/D-tyrosine methyl esters, in this case as their phenolate salts. The oxidation of β-naphthol has also been studied as a model monooxygenase reaction. The catechol oxidation was investigated in a range of substrate concentrations at slightly acidic pH and exhibited a marked dependence on the concentration of the [Cu2EHI]4+ complex. This behavior has been interpreted in terms of an equilibrium between a monomeric and a dimeric form of the catalyst. Binding studies of L- and D-tyrosine were performed as a support for the interpretation of the stereoselectivity effects observed in the reactions. In general, [Cu2EHI]4+ exhibits a binding preference for the L- rather than the D-enantiomer of the substrates, but it appears that in the catecholase reaction the oxidation of the D-isomer occurs at a faster rate than for the L counterpart. The same type of enantio-discriminating behavior is observed in the oxidation of L-/D-tyrosine methyl esters. In this case the reaction produces a complex mixture of products; the main product consisting of a trimeric compound, likely formed by radical coupling reactions, has been isolated and characterized. The oxidation of β-naphthol yields an additional product of the expected quinone but labeling experiments with 18-O2 show no oxygen incorporation into the product, confirming that the oxidation likely proceeds through a radical mechanism.


 Please wait while we load your content...
Please wait while we load your content...