Inner to outer-sphere coordination of plutonium(iv) with N,N-dialkyl amide: influence of nitric acid†
Abstract
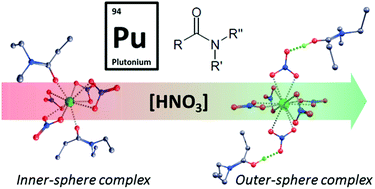
N,N-Dialkylamides are extensively studied as alternative organic ligands to achieve the extraction and separation of uranium(VI) and plutonium(IV). We report here the coordination structures of the plutonium(IV) ion with N,N-di(2-ethylhexyl)-n-butanamide as a function of nitric acid concentration in the aqueous phase. The coordination structure of Pu(IV) evolves gradually with increasing nitric acid concentration from an inner-sphere with two coordinated amide ligands toward an outer-sphere hexanitrate complex with only nitrate ions in the first coordination sphere and protonated amide ligands in the outer shell.


 Please wait while we load your content...
Please wait while we load your content...