A study of asymmetrical mixed-valent Mo2–Mo2 complexes in the class III regime†
Abstract
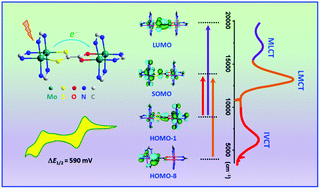
Three novel asymmetrical dimolybdenum dimers, [Mo2(DAniF)3]2(μ-OOCCOS) (DAniF = N,N′-di(p-anisyl)formamidinate) ([OO–OS]), [Mo2(DAniF)3]2(μ-S2CCO2) ([SS–OO]), and [Mo2(DAniF)3]2(μ-SSCCOS) ([SS–OS]), have been synthesized and characterized by either single-crystal X-ray crystallography or 1H NMR spectroscopy. The structural asymmetry for these compounds gives rise to a redox asymmetry, which enlarges the potential separation (ΔE1/2) between the two [Mo2] units. The mixed-valance (MV) species [OO–OS]+, [SS–OO]+ and [SS–OS]+, prepared by one-electron chemical oxidation of the neutral precursors, exhibit an intense and symmetrical intervalence charge transfer (IVCT) absorption band in the near-IR region, along with the high energy metal (δ) to ligand (π*) (ML) and ligand (π) to metal (δ) charge transfer (LMCT) absorptions. The LMCT band, which is absent in the neutral precursors, is reflective of the cationic [Mo2]+ unit in the MV species; therefore, it is evidenced that in the MV complexes optical electron transfer from the electron donor to acceptor occurs, while the thermal process is energetically unfavorable. The C(1)–C(2) bonds (1.44–1.48 Å) that connect the two [Mo2] units are significantly shorter than a C–C single bond, showing that the two Mo2 centers are strongly coupled. For the series, TD-DFT calculations show that the molecular orbitals have an unsymmetrical charge density distribution over the two dimolybdenum sites. For each of the complex systems, the calculated orbital energy gaps, SOMO(δ − δ)–LUMO(bridging ligand π*), HOMO−8(bridging ligand π)–SOMO(δ − δ) and SOMO(δ − δ)–HOMO−1(δ + δ), are in good agreement with the observed MLCT, LMCT and IVCT absorption band energies, respectively. The consistency in energy between the IVCT band and the SOMO(δ − δ)–HOMO−1(δ + δ) gap permits assignment of the MV complexes to Class III in the Robin–Day scheme.
- This article is part of the themed collection: Multimetallic complexes: synthesis and applications


 Please wait while we load your content...
Please wait while we load your content...