Electrochemical characterization of plutonium in n-tributyl phosphate†
Abstract
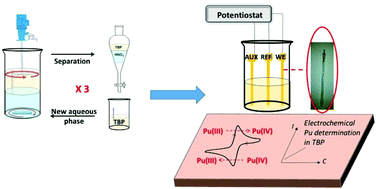
This work investigates the electrochemical behavior of Pu(IV) and Pu(VI) complexes in n-tributylphosphate (TBP) as an entry to the electrochemical characterization of these complexes in organic extractants related to nuclear fuel reprocessing. Glassy carbon electrodes were used to show that Pu(IV) and Pu(VI) complexes display a reversible electrochemical reduction wave in TBP previously equilibrated with aqueous nitric acid solution. We investigated the reduction of Pu(IV) and Pu(VI) nitrato complexes extracted into TBP, with the aim to get thermodynamic (formal potential) and kinetic (diffusion coefficient) information about Pu(IV)/Pu(III) and Pu(VI)/Pu(V) redox couples in the TBP medium. The formal potentials of the two redox couples were respectively 0.510 ± 0.005 and 0.478 ± 0.005 V per SCE in TBP equilibrated with 3 mol L−1 nitric acid at room temperature. The diffusion coefficient values of Pu(IV) and Pu(VI) species were estimated to be 0.72 × 10−6 and 0.77 × 10−6 cm2 s−1 respectively. Also, the Pu(IV) reduction showed a Nernstian dependence on the logarithm of nitric acid concentration in the organic phase, featuring the exchange of nitrates upon reduction of Pu(IV).


 Please wait while we load your content...
Please wait while we load your content...