Enantiopure and racemic radical-cation salts of bis(2′-hydroxylpropylthio)(ethylenedithio)TTF with polyiodide anions†
Abstract
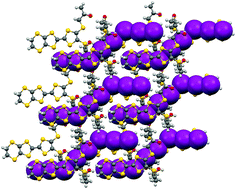
The chiral TTF-based donor molecule bis(2′-hydroxylpropylthio)(ethylenedithio)tetrathiafulvalene (BHPT-EDT-TTF) has produced enantiopure R,R and S,S radical-cation salts with polyiodide anions I3− and I82−. Enantiomorphic 6 : 6 donor : I3 phases grown from either the R,R or S,S donor are semiconducting with similar activation energies of 0.24–0.30 eV and 0.22–0.23 eV, respectively, and contain three unique face-to-face donor pairs whose relative orientation is determined by side chain conformations and hydrogen bonding. Racemic material under the same conditions gave an insulating centrosymmetric phase with R,R and S,S donor cations in a face-to-face pair partnered with an octaiodide dianion, and with a ca. 3 : 1 disorder between the enantiomers. Enantiopure BHPT-EDT-TTF yielded two further insulating crystalline phases of composition 2 : 2 with triiodide and 2 : 1 with octaiodide.


 Please wait while we load your content...
Please wait while we load your content...