Diastereoselective silylene transfer reactions to chiral enantiopure alkenes: effects of ligand size and substrate bias†
Abstract
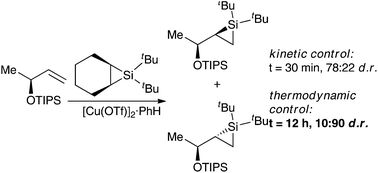
Silylenes are useful reactive intermediates for the stereoselective construction of compounds containing carbon–silicon bonds. Despite their synthetic utility, the development of either an enantioselective or diastereoselective metal-catalyzed silylene transfer reaction, in which ligands on the metal catalyst control stereoselectivity, has not been achieved. In this article, we report that the structure of the alkene is the most important for controlling stereoselectivity in these reactions. The stereochemical course of kinetically controlled silacyclopropanation reactions was not affected by the nature or chirality of the ligands on the metal. When silylene transfer reactions were reversible, however, products can be formed with a high degree of diastereoselectivity (90 : 10 d.r.).
- This article is part of the themed collection: Silicon Chemistry: Discoveries and Advances


 Please wait while we load your content...
Please wait while we load your content...