Interaction of copper with dinitrogen tetroxide in 1-butyl-3-methylimidazolium-based ionic liquids†
Abstract
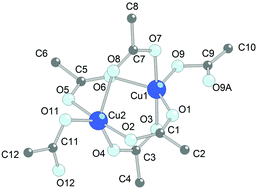
Ionic liquids that are stable toward oxidation and nitration and are based on the 1-n-butyl-3-methylimidazolium cation (BMIm+) can be used as solvents and reaction media for copper dissolution in liquid dinitrogen tetraoxide N2O4. The ionic liquid not only favors the dissociation of N2O4 into NO+ and NO3−, but also takes part in the formation of different crystalline products. Thus, NO[BF4], NO[Cu(NO3)3] and (BMIm)2[Cu2(CF3COO)6] were prepared using (BMIm)A, A− = [BF4]−, (CF3SO2)2N−, CF3COO−, respectively. The formation of a certain product is determined by the nature of the anion A− and the relative solubility of the reaction products in the ionic liquid. Crystals of NO[BF4] were also prepared directly from a mixture of N2O4 and BMImBF4. According to XRD single-crystal structure analysis, the structure of NO[BF4] consists of tetrahedral [BF4]− anions and nitrosonium NO+ cations; the formation of these ions prove the heterolytic dissociation of N2O4 dissolved in the ionic liquid. The crystal structure of the earlier unknown binuclear copper trifluoroacetate (BMIm)2[Cu2(CF3COO)6] were determined by X-ray diffraction. The peculiarity of this dimer compared to the majority of known dimeric copper(II) carboxylates is the unusually long Cu⋯Cu distance (3.15 Å), with Cu(II) ions demonstrating an atypical coordination of a distorted trigonal bipyramid formed by five O atoms of five trifluoroacetate groups.


 Please wait while we load your content...
Please wait while we load your content...