Eight-membered-ring diaminocarbenes bearing naphthalene moiety in the backbone: DFT studies, synthesis of amidinium salts, generation of free carbene, metal complexes, and solvent-free copper catalyzed azide–alkyne cycloaddition (CuAAC) reaction†
Abstract
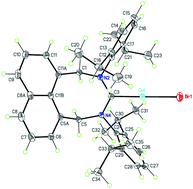
A new type of eight-membered ring N-heterocyclic carbene (NHC) bearing a rigid naphthalene moiety in the backbone is reported for the first time. Stereoelectronic properties of 4,5-dihydro-1H-naphtho[1,8-ef][1,3]diazocin-3(2H)-ylidene (NaphtDHD) and smaller ring NHCs were theoretically studied at the DFT level. Amidinium salts were prepared from corresponding amidines and dibromides. Free carbene NaphtDHD-Dipp (Dipp = 2,6-diisopropylphenyl) was generated in solution by treatment of the corresponding salt with LiHMDS. It is stable in solution at low temperatures, while decomposing rapidly at room temperature. Silver(I) and copper(I) complexes were synthesized and structurally characterized in the solid state. The copper(I) complex [(NaphtDHD-Mes)CuBr] (Mes = mesityl, 2,4,6-trimethylphenyl) exhibits high catalytic activity in alkyne–azide cycloaddition (CuAAC) reaction under solvent-free conditions.


 Please wait while we load your content...
Please wait while we load your content...