Abstract
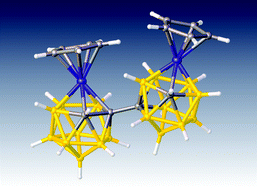
Double deboronation of 1,1′-bis(ortho-carborane) results in a mixture of racemic and meso diastereoisomers which are sources of the [7-(7′-7′,8′-nido-C2B9H10)-7,8-nido-C2B9H10]4− tetraanion. Consistent with this, metalation of the mixture with {Ru(p-cymene)} affords the diastereoisomers α-[1-(8′-2′-(p-cymene)-2′,1′,8′-closo-RuC2B9H10)-3-(p-cymene)-3,1,2-closo-RuC2B9H10] (3α) and β-[1-(8′-2′-(p-cymene)-2′,1′,8′-closo-RuC2B9H10)-3-(p-cymene)-3,1,2-closo-RuC2B9H10] (3β) in which the primed cage has undergone a spontaneous 3′,1′,2′ to 2′,1′,8′-RuC2B9 isomerisation. Analogous cobaltacarboranes α-[1-(8′-2′-Cp-2′,1′,8′-closo-CoC2B9H10)-3-Cp-3,1,2-closo-CoC2B9H10] (4α) and β-[1-(8′-2′-Cp-2′,1′,8′-closo-CoC2B9H10)-3-Cp-3,1,2-closo-CoC2B9H10] (4β) are formed by metalation with CoCl2/NaCp followed by oxidation, along with a small amount of the unique species [8-(8′-2′-Cp-2′,1′,8′-closo-CoC2B9H10)-2-Cp-2,1,8-closo-CoC2B9H10] (5) if the source of the tetraanion is [HNMe3]2[7-(7′-7′,8′-nido-C2B9H11)-7,8-nido-C2B9H11]. Two-electron reduction and subsequent reoxidation of 4α and 4β afford species indistinguishable from 5. The reaction between [Tl]2[1-(1′-3′,1′,2′-closo-TlC2B9H10)-3,1,2-closo-TlC2B9H10] and [CoCpI2(CO)] leads to the isolation of a further isomer of (CpCoC2B9H11)2, rac-[1-(1′-3′-Cp-3′,1′,2′-closo-CoC2B9H10)-3-Cp-3,1,2-closo-CoC2B9H10] (6), which displays intramolecular dihydrogen bonding. Thermolysis of 6 yields 4α, allowing a link to be established between the α and β forms of 3 and 4 and racemic and meso forms of the [7-(7′-7′,8′-nido-C2B9H10)-7,8-nido-C2B9H10]4− tetraanion, whilst reduction–oxidation of 6 again results in a product indistinguishable from 5.


 Please wait while we load your content...
Please wait while we load your content...