Organometallic cobalamin anticancer derivatives for targeted prodrug delivery via transcobalamin-mediated uptake†
Abstract
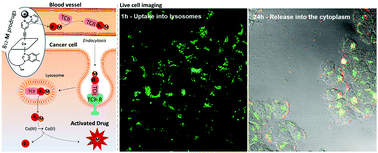
Herein we report the synthesis of new water-soluble vitamin B12 prodrugs bearing metal complexes at the β-upper side of the cobalt center. A total of three derivatives with the general design {Co–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C-bpy–M}, where M represents a cytotoxic metal complex, were prepared and tested for their cytotoxicity against MCF-7 breast cancer cells. The choice of the metal was oriented on the eminent Pt and promising Ru and Re species to demonstrate the general applicability of the approach. The recognition of the derivatives by transcobalamin was demonstrated by competitive displacement assays using rhodamine labeled B12. This compound further served to prepare a dual luminescent probe by orthogonal synthesis with M = ((HCCbpy)Ru(bpy)2)Cl2 and to perform in vitro assays. Cellular imaging experiments allowed us to observe the different compartmentalization of both dyes and thus prove that the species follow the natural cobalamin uptake as well as the self-triggered release of the β-upper complex.
C-bpy–M}, where M represents a cytotoxic metal complex, were prepared and tested for their cytotoxicity against MCF-7 breast cancer cells. The choice of the metal was oriented on the eminent Pt and promising Ru and Re species to demonstrate the general applicability of the approach. The recognition of the derivatives by transcobalamin was demonstrated by competitive displacement assays using rhodamine labeled B12. This compound further served to prepare a dual luminescent probe by orthogonal synthesis with M = ((HCCbpy)Ru(bpy)2)Cl2 and to perform in vitro assays. Cellular imaging experiments allowed us to observe the different compartmentalization of both dyes and thus prove that the species follow the natural cobalamin uptake as well as the self-triggered release of the β-upper complex.


 Please wait while we load your content...
Please wait while we load your content...