An unprecedented photochromic system with cis-oriented dithienyl-dithiolenes supported by metal chelation†
Abstract
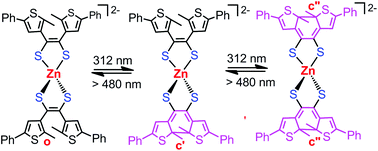
4,5-Bis(2-methyl-5-phenylthiophen-3-yl)-1,3-dithiol-2-one (L1o) was elaborately designed to afford dithienyl-dithiolene as a new photochromic ligand. We describe herein the preparation and characterization of unprecedented photochromic dithienyl-dithiolene complexes with cis-orientation of dithienylethene (DTE) stabilized by metal chelation instead of conventional cyclopentene. The treatment of L1o with sodium methoxide in methanol afforded a disodium salt of dithiolate dianion, which reacts with M(dppe)Cl2 (dppe = 1,2-bis(diphenylphosphino)ethane, M = Ni, Pd) to give neutral compounds M(dppe)(dithiolate) as established by X-ray crystallography. The reaction of L1o with NiCl2 in the presence of sodium methoxide allows the isolation of an anionic nickel(II) bis(dithienyl-dithiolene) complex with photochemical inertness. In contrast, the corresponding reaction with ZnCl2 afforded a dianionic zinc(II) complex chelated by two dianionic dithienyl-dithiolenes, which displays stepwise photocyclization upon irradiation with UV light at 312 nm as demonstrated experimentally and theoretically. Only when dithienyl-dithiolene behaves as a dicationic ligand instead of neutral or monoanionic species, it is possible to achieve reversible photochromism in the corresponding metal complexes.


 Please wait while we load your content...
Please wait while we load your content...