A structural study of alkaline earth metal complexes with hybrid disila-crown ethers†
Abstract
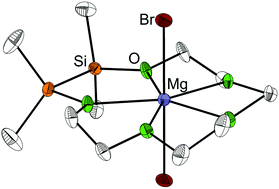
Compounds consisting of [M(1,2-disila-[3n]crown-n)]2+ (M = Mg, Ca, Sr, Ba; n = 5, 6) and [Ba(1,2-disila-benzo[18]crown-6)]2+ cations and different anions were obtained by equimolar reaction of the hybrid disila-crown ethers 1,2-disila[15]crown-5 (1), 1,2-disila[18]crown-6 (2) and 1,2-disila-benzo[18]crown-6 (7) with alkaline earth metal salts. Even with strongly coordinating anions such as Br− or I− stable complexes could be obtained, showing the good coordination ability of these ligands. The structures of all coordination compounds were determined via single crystal X-ray diffraction (XRD). By means of DFT calculations, the complexation ability of 1,2-disila[15]crown-5 (1) towards magnesium bromide was determined to be considerably higher compared to [15]crown-5. The opposite case was observed in solution as the exchange of calcium cations between [18]crown-6 and 1,2-disila[18]crown-6 (2) was studied via dynamic proton nuclear magnetic resonance (NMR) spectroscopy.
- This article is part of the themed collection: Silicon Chemistry: Discoveries and Advances

 Please wait while we load your content...
Please wait while we load your content...