Geometrical properties of the manganese(iv)/iron(iii) cofactor of Chlamydia trachomatis ribonucleotide reductase unveiled by simulations of XAS spectra†
Abstract
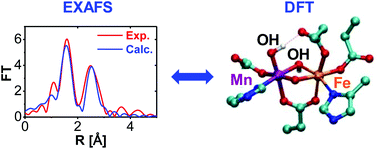
Using simulations of Mn/Fe K-edge X-ray absorption spectroscopy (XAS), combined with DFT-optimized structural models and direct comparisons with available experimental data, we determine geometrical and electronic properties of the Mn–Fe active site of Chlamydia trachomatis (Ct) of ribonucleotide reductase (RNR). In the post-edge XAS energy range, we use extended X-ray absorption fine structure (EXAFS) data, to acquire absorber–scatterer geometrical information around each absorber metal center. For this task, we apply a protocol that evaluates Debye–Waller factors in scattering paths instead of scattering shells to fit the experimental EXAFS. The model of the manganese(IV)/iron(III) cofactor that best fit Mn and Fe K-edge EXAFS experimental data is a structure with Mn at metal site 1 (proximal to Phe-127), a μ-oxo/μ-hydroxo/μ-1,3-carboxylato core, and a terminal hydroxo ligand, i.e. OH−–Mn1(IV)–(μ-O)(μ-OH−)-Fe2(III).


 Please wait while we load your content...
Please wait while we load your content...