A new route for the preparation of enriched iso-polylactide from rac-lactide via a Lewis acid catalyzed ring-opening of an epoxide†
Abstract
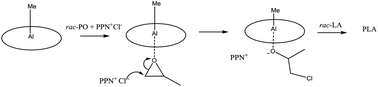
Tetraphenylporphyrin aluminum(III) salts, TPPAlX, where X = Me, OEt, OiPr, OCHMeCH2Cl, and Cl, and bis(triphenylphosphine)imminium chloride, PPN+Cl− (1 : 1) react with rac-lactide, rac-LA, in neat propylene oxide, PO, to yield chains of enriched isotactic polylactide, PLA, with end groups of PO–Cl and with time these yield cyclic polymers (PO)n(PLA) where n = 2 or 3 and even higher. There is no reaction between TPPAlOR (R = Et or iPr), PPN+Cl−, and rac-LA in neat THF at 25 °C even though TPPAlOR (R = Et or iPr) and PPN+Cl− in neat PO yields polypropylene oxide with a terminal OR group, H(PO)nOR. Taken together, Al(III) acts as a Lewis acid in the ring-opening of PO, in which PPN+Cl− is present and the incipient ClCH2CHMeO− initiates the ROP of LA to yield anion chains of [(PLA)-OCHMeCH2Cl]−, and then the ring-opening of PO yields cycles, (PO)n(PLA), with the liberation of Cl−. The polymer was isolated by the addition of MeOH/HCl and end group analysis by mass spectrometry.


 Please wait while we load your content...
Please wait while we load your content...