Fluorescent H-aggregates of an asymmetrically substituted mono-amino Zn(ii) phthalocyanine†
Abstract
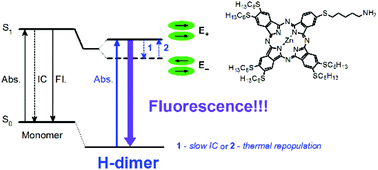
The photophysical properties of a newly synthesized unsymmetrically substituted zinc phthalocyanine derivative (1) bearing in its peripheral positions six n-hexylsulfanyl substituents and one amino-terminated n-hexylsulfanyl substituent were investigated. This mono-amino phthalocyanine exhibited a high tendency to form H-type aggregates in all of the investigated solvents: dichloromethane (DCM), tetrahydrofuran (THF) and dimethyl sulfoxide (DMSO). Several species of H-aggregates were present together in relatively broad concentration ranges in THF and DCM, whereas in DMSO they were observed separately depending on the concentration used. Despite the widely accepted non-emissive character of H-type dimers, the H-type aggregates of phthalocyanine 1 were highly emissive in all solvents: the fluorescence quantum yield in DMSO for the n-aggregate is equal to 0.05, whereas for the (n + 1)-aggregate it is 0.11. Upon (n + 1)-aggregation, the fluorescence lifetime of the n-aggregate increased from ca. 2.5 ns to 3.3 ns. Based on these results, the radiative lifetimes of both species were computed: 48 ns for the n-aggregate and 29 ns for the (n + 1)-aggregate. The determined oscillator strengths for the n-aggregate and the (n + 1)-aggregate in DMSO were 0.04 and 0.12, respectively. The observed emission of the H-type (n + 1)-aggregate was assigned to the radiative transition from the upper exciton state to the ground state, which could be rationalized by a constant thermal repopulation of the upper exciton state. The experimental findings were supported by theoretical calculations.


 Please wait while we load your content...
Please wait while we load your content...