Zeolite-supported rhodium sub-nano cluster catalyst for low-temperature selective oxidation of methane to syngas†
Abstract
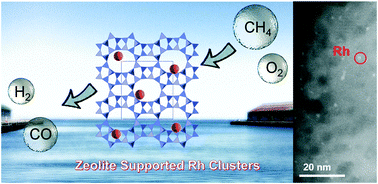
The conversion of methane to syngas under mild conditions is a key to efficiently utilise natural gas, including shale gas, in chemical industries. In this work, we demonstrate that Rh sub-nano clusters formed on zeolites catalyse the partial oxidation of methane to syngas at substantially lower temperatures (450–600 °C) than those required in previous reports (>700 °C). Rh sub-nano clusters of 0.6 nm diameter were prepared by a simple ion-exchange method. The catalyst gave 84% conversion of methane with 91% selectivity for CO and a H2/CO ratio of 2.0 at 600 °C even at a very high space velocity (1 200 000 mL h−1 g−1). No deactivation was observed in the durability test for 50 h, and the turnover number of bulk Rh for the formation of CO reached 2 600 000. Kinetic and spectroscopic studies revealed that a combustion-reforming mechanism occurs on the metallic Rh sub-nano clusters. The acidity of the zeolite played no role in the reaction, but the ion-exchange properties of zeolites were crucial to prepare the active Rh catalysts.


 Please wait while we load your content...
Please wait while we load your content...