Selective conversion of lactic acid to acrylic acid over alkali and alkaline-earth metal co-modified NaY zeolites†
Abstract
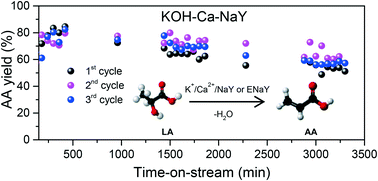
Alkali and alkaline-earth metal cation co-modified NaY zeolites were systematically synthesized and comprehensively investigated as catalysts for gas-phase dehydration of lactic acid (LA) to acrylic acid (AA). The long-term (time-on-stream >55 h) catalytic performance in four repeated reaction–regeneration cycles was studied. The best performing catalyst shows a consistently high AA selectivity of ∼84% at different weight hourly space velocity (WHSV) values ranging from 0.48 to 4.8 h−1. Most importantly, the catalyst can still deliver a high AA selectivity of ∼82% after four long-term reaction–regeneration cycles. The investigation shows that mild etching increases the defect density of the zeolite and thus leads to poor hydrothermal stability in the long-term reaction–regeneration cycles. The strong acidic adsorbate/catalyst surface interaction (base property) and the acidity of the catalyst are responsible for the catalyst deactivation. The role of the alkali and alkaline-earth metal cations and the transformation of these cations during the reaction and regeneration process are presented.


 Please wait while we load your content...
Please wait while we load your content...