Modelling complete methane oxidation over palladium oxide in a porous catalyst using first-principles surface kinetics†
Abstract
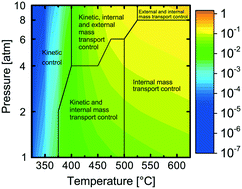
A comprehensive model is developed for complete methane oxidation over supported palladium. The model is based on first-principles microkinetics and accounts for mass and heat transport in a porous catalytic layer. The turnover frequency (TOF) is simulated for wet exhaust gas compositions, exploring the effects of temperature and total pressure on the TOF. Three different temperature regimes are identified each with different dependency on the total pressure. The regimes originate from temperature and pressure dependent coverages of carbon dioxide and water, which are the most abundant surface species hindering methane dissociation at low temperatures. The TOF is controlled by surface kinetics below 400 °C whereas above 500 °C and up to 8 atm, internal mass transport is controlling. A combination of kinetics, external and internal mass transport controls the TOF at other reaction conditions. The physically meaningful model paves the way for extrapolation and optimization of catalyst design parameters for high catalytic efficiency.


 Please wait while we load your content...
Please wait while we load your content...