Mechanisms of metal-catalyzed cycloisomerizations of o-propargylbiaryls and o-allenylbiaryls to phenanthrenes: a DFT study†
Abstract
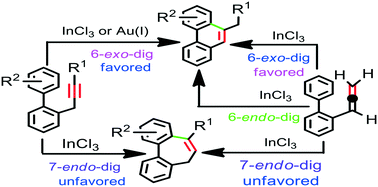
A theoretical investigation of the mechanism and regioselectivity of the InCl3- and Au(I)-catalyzed cycloisomerization of o-propargylbiphenyl as well as the InCl3-catalyzed cycloisomerization of o-allenylbiphenyl has been carried out with the aid of DFT calculations. The calculation results reveal that the Au(I)-catalyzed cycloisomerization of o-propargylbiphenyl occurs exclusively via 6-exo-dig cyclization in a Friedel–Crafts-type mechanism. The results also indicate that the 6-exo-dig cyclization is highly favored over the 7-endo-dig cyclization with InCl3 catalyst, while the mechanism via allene intermediate is less likely. In the Au(I) catalysis, the Friedel–Crafts-type intermediate is converted to the exo-double bond intermediate by a direct 1,3-H shift and then stepwise two consecutive 1,2-H shift steps leading to the final product. However, in the InCl3 catalysis intermolecular chloride-assisted H-abstraction/proto-demetalation between two Friedel–Crafts-type intermediates are more favorable than the direct 1,3-H shift. In addition, intramolecular chloride-assisted H-abstraction/proto-demetalation are more favorable than the stepwise two consecutive 1,2-H shift steps for the transformation of the exo-double bond intermediate into the final product. The feasibility of the InCl3-catalyzed cycloisomerization of o-propargylbiphenyl depends on the nature of the substituent on the phenyl ring and/or in the propargyl moiety. It is found that the cycloisomerization of electron-deficient o-propargylbiaryl as well as an unfunctionalized biaryl bearing alkyl group in the propargyl moiety may not be feasible. In contrast, InCl3-catalyzed cycloisomerization of o-propargylbiphenyl is both kinetically and thermodynamically more favorable than that of o-allenylbiphenyl. The cycloisomerization of o-allenylbiphenyl prefers 6-exo-dig over 6-endo-dig and 7-endo-dig cyclizations. Our calculation results are in good agreement with the experimental observations.


 Please wait while we load your content...
Please wait while we load your content...