Reaction pathway for partial hydrogenation of 1,3-butadiene over Pt/SiO2†
Abstract
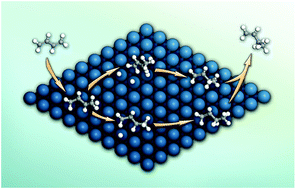
The reaction pathway for partial hydrogenation of 1,3-butadiene over a Pt/SiO2 catalyst was explored with a combination of in situ diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy, intrinsic kinetics, and density functional theory (DFT) calculations. Under the present experimental conditions, the catalyst displayed a nearly constant product composition with ∼97% selectivity to butenes. In situ DRIFT characterization revealed that the 1-buten-3-yl radical (1B3R), generated from the addition of one hydrogen atom to a terminal carbon of 1,3-butadiene, was the dominant intermediate in the partial hydrogenation of 1,3-butadiene. Kinetic analysis showed that the hydrogenation of 1B3R was the rate-determining step in the formation of butenes. Based on the above experimental results, DFT calculations were employed to investigate the reaction pathway with 1B3R as the intermediate on a Pt(111) surface. Interestingly, it was found that 1B3R can be easily formed from the hydrogenation of 1,3-butadiene with a di-σ configuration rather than the most stable tetra-σ structure on the Pt(111) surface. This hydrogenation step occurs between the non-coordinated terminal carbon and a hydrogen atom on a top site with an energy barrier of 23.2 kJ mol−1. The second hydrogenation step from 1B3R to butenes requires relatively higher activation barriers to proceed, being consistent with the experimental kinetics. Finally, the selectivity order for butenes and the structure sensitivity of Pt-catalyzed partial hydrogenation of 1,3-butadiene were discussed.


 Please wait while we load your content...
Please wait while we load your content...