Dissolution induced self-selective Zn- and Ru-doped TiO2 structure for electrochemical generation of KClO3†
Abstract
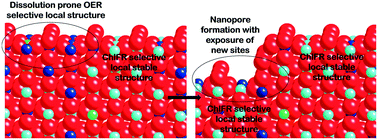
We demonstrate an electrochemical approach to prepare a highly active and stable (Zn, Ru)-doped TiO2 (Ru0.26Ti0.73Zn0.01Ox) for electrochemical generation of KClO3. The essential ingredients of this approach consists of (1) co-electrodeposition of Ru, Ti, and Zn as a metallic alloy and then annealing it to form an oxide, (2) dissolution under electrochemical oxidative acidic environment of Ru and Zn-rich surface clusters having a high selectivity towards oxygen evolution reaction (OER) affording the Ti-enriched structure that is more selective to chlorine evolution reaction (CER), and (3) using this electrocatalyst for KClO3 production under near-neutral pH conditions. The dissolution of Ru- and Zn-enriched surfaces resulted in a porous structure with a higher electrochemical surface area and roughness. Furthermore, this electrochemical dissolution of the (Zn, Ru)-rich surface results in an electrocatalytic structure that is self-selective towards the KClO3 formation with a higher specific activity. The co-electrodeposition of Zn aids (1) the formation of the roughened and porous surface structure through surface dissolution of Zn- or ZnO-clusters and (2) the enhancement of conductivity of the electrocatalyst through the formation of oxygen vacancies. The obtained structure exhibited a higher specific activity and stability towards the KClO3 production in comparison to the untreated (Zn, Ru)-doped TiO2 or Ru-doped TiO2 or RuO2.


 Please wait while we load your content...
Please wait while we load your content...