A new d-threonine aldolase as a promising biocatalyst for highly stereoselective preparation of chiral aromatic β-hydroxy-α-amino acids†
Abstract
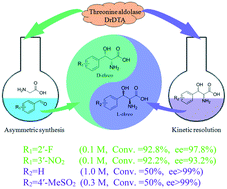
D-Threonine aldolase is an enzyme belonging to the glycine-dependent aldolases, and it catalyzes the reversible aldol reaction of glycine and acetaldehyde to give D-threonine and/or D-allo-threonine. In this study, a putative D-threonine aldolase gene from Delftia sp. RIT313 was cloned and expressed in Escherichia coli BL21 (DE3). The purified enzyme (DrDTA, 47 KDa) exhibited 21.3 U mg−1 activity for the aldol addition of glycine and acetaldehyde in MES-NaOH buffer (pH 6.0) at 50 °C. Both pyridoxal 5′-phosphate and metal ions were needed for the reaction, and the existence of the metal ions enhanced the stability of the enzyme. It was found that the conversion and Cβ-stereoselectivity were dramatically influenced by the reaction temperature, co-solvent, amount of enzyme and reaction time, and it is possible to enable the reaction under kinetic control to retain suitable conversion and high stereoselectivity at the β-carbon, thus tackling the “Cβ-stereoselectivity problem”. DrDTA showed high activity toward aromatic aldehydes with electron-withdrawing substituents. Under the optimized reaction conditions, phenylserines with a 2′-fluoro- or 3′-nitro-substituent were obtained with >90% conversion and >90% de. In addition, DL-threo-phenylserine and DL-threo-4-(methylsulfonyl)phenylserine were efficiently resolved with an excellent enantiomeric excess value (ee, >99%) using a whole cell biocatalyst in a two-phase system at 1.0 M and 0.3 M, respectively, the highest substrate concentration reported so far. These results suggested that DrDTA might be a promising biocatalyst for producing chiral aromatic β-hydroxy-α-amino acids.


 Please wait while we load your content...
Please wait while we load your content...