Improving Pseudomonas fluorescens esterase for hydrolysis of lactones†
Abstract
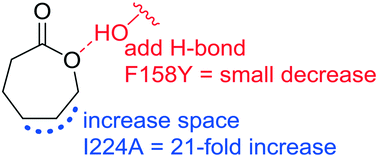
Although both acyclic esters and lactones contain ester functional groups, their shapes differ and most esterases are poor catalysts for hydrolysis of lactones. For example, hydrolysis of β-butyrolactone, α-angelicalactone and ε-caprolactone catalyzed by esterase from Pseudomonas fluorescens (PFE) is 10, 48, and 510 times slower than hydrolysis of the ester p-nitrophenol acetate (kcat/Km = 26 000 M−1 s−1). Two possible reasons for the slower hydrolysis of lactones by PFE are the different orientations of 1) the alcohol oxygen lone pairs (mechanistic hypothesis) or 2) the alcohol alkyl group in lactones (shape hypothesis). To test these hypotheses, we engineered higher lactonase activity into esterase PFE using site-directed mutagenesis. First, we added a hydrogen bond donor near the lactone alcohol oxygen (F158Y), but these variants showed no significant increase (p = 0.44) in lactonase catalytic efficiency with ε-caprolactone (17 ± 24 fold increase over 9 variants) as compared to variants without this substitution (10 ± 7 fold increase over 28 variants). Docking placed the hydroxyl group of Tyr158 too far from the lactone to hydrogen bond suggesting that additional substitutions would be needed to create this interaction. Second, we created space for the lactone alcohol alkyl group (I224A). These variants showed a significant increase (p < 0.001) in lactonase catalytic efficiency (32 ± 16 fold over 13 variants) as compared to variants without this substitution (1.2 ± 0.5 fold over 14 variants). Docking placed ε-caprolactone within hydrogen bonding distance of the catalytic histidine in the PFE I224A variant, while it was too far in wild-type PFE. The best combination of substitutions for ε-caprolactone and β-butyrolactone was L29P/F143V/F158Y/I224A, whose kcat/Km was 86 and 13 times higher, respectively, than wt PFE. For α-angelicalactone, the best variant was F143W/I224A, which increased kcat/Km 20-fold over wt PFE. These results suggest that matching the shape of the binding site to the shape of the lactone is the most effective way to increase lactonase activity in esterases.


 Please wait while we load your content...
Please wait while we load your content...