Reactions catalyzed by a binuclear copper complex: selective oxidation of alkenes to carbonyls with O2†
Abstract
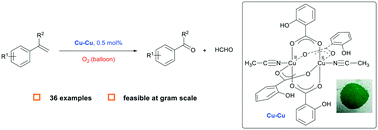
Terminal alkenes were selectively cleaved into ketones and aldehydes catalyzed by a binuclear copper catalyst bearing a simple salicylate ligand with O2 as the oxidant. The reaction was carried out under an atmosphere of O2 (balloon) with 0.5 mol% of catalyst and could be performed on a gram scale, providing a convenient and practical method for the cleavage of terminal alkenes into carbonyl compounds.


 Please wait while we load your content...
Please wait while we load your content...