Fe–Zr–O catalyzed base-free aerobic oxidation of 5-HMF to 2,5-FDCA as a bio-based polyester monomer†
Abstract
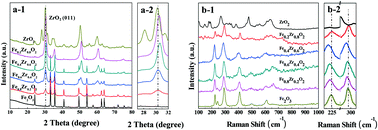
An environment-friendly and economical route for 5-hydroxymethylfurfural (HMF) aerobic oxidation to 2,5-furandicarboxylic acid (FDCA) in an ionic liquid (IL)-promoted base-free reaction system was reported using Fe–Zr–O as a catalyst. A series of FexZr1−xO2 catalysts were synthesized by a hydrothermal method and the catalytic performance was investigated. Among these catalysts, Fe0.6Zr0.4O2 exhibited excellent catalytic activity in the HMF oxidation. A 60.6% FDCA yield and 99.9% HMF conversion could be obtained after 24 h under 2 MPa O2 pressure and base-free conditions. The good performance could be attributed to the large amount of acidic and basic sites on the surface of the catalyst and its high reducibility and oxygen mobility. In addition, the formation of humins and the reaction pathways in the ILs were also investigated, which revealed parallel reactions between FDCA and humin formation. A plausible reaction mechanism was proposed based on the results of a series of designed experiments. Finally, the catalyst was used five times without obvious loss of activity. To the best of our knowledge, this is the best result for a non-noble metal catalyzed base-free oxidation of HMF to FDCA using molecular oxygen as an oxidant.


 Please wait while we load your content...
Please wait while we load your content...