Enantioselective hydrogenation of N-heteroaromatics catalyzed by chiral diphosphine modified binaphthyl palladium nanoparticles†
Abstract
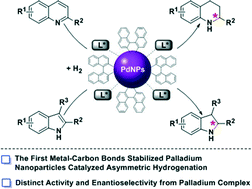
The first application of binaphthyl-stabilized palladium nanoparticles (Bin-PdNPs) with chiral modifiers in asymmetric hydrogenation of N-heteroaromatics is revealed. With an appropriate ratio of R-BINAP/Bin-PdNPs used, the pre-prepared chiral nanocatalyst achieves asymmetric hydrogenations of 2-substituted quinolines with good to excellent yields and moderate enantioselectivities, which showed superior catalytic properties to the R-BINAP/Pd complex. Moreover, this protocol is also applicable to 2-substituted indoles.


 Please wait while we load your content...
Please wait while we load your content...