Actualizing efficient photocatalytic water oxidation over SrTaO2N by Na modification†
Abstract
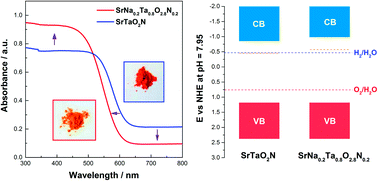
Despite a strong visible light absorption at a wavelength as far as 610 nm, SrTaO2N generally exhibits poor photocatalytic activity due to photocatalytic self-decomposition. In this study, we introduce Na into the B site of SrTaO2N and perform an investigation on its crystal structure, optical absorption and photocatalytic water oxidation in response to Na incorporation. Our results suggest that Na incorporation effectively enhances local Ta–O(N) bond strength, which is responsible for strong interband light absorption, low tendency to defect formation and high stability against photocatalytic self-decomposition. Efficient photocatalytic water oxidation was realized in Na-modified SrTaO2N (Sr0.8Na0.2TaO2.8N0.2) with an apparent quantum efficiency as high as 0.2% under visible light illumination (≥400 nm). This simple strategy highlights the benefits of alkaline metal modification and can be extended to other nitrides or oxynitrides where management of local bond strength is needed.


 Please wait while we load your content...
Please wait while we load your content...