Direct and selective hydrogenation of CO2 to ethylene and propene by bifunctional catalysts†
Abstract
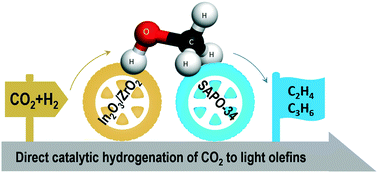
Reduction of CO2 by H2 produced from renewable electricity on a large scale would benefit both carbon recycling as well as H2 storage and transport. Among the various CO2 hydrogenation reaction products, light olefins, such as ethylene and propylene, are very important intermediates in the chemical industry. However, very efficient catalytic systems that are able to drive CO2 hydrogenation reactions selectively to make olefins do not exist although the reactions are thermodynamically favorable. In this study, we demonstrated a selective hydrogenation process to directly convert CO2 to light olefins via a bifunctional catalyst composed of a methanol synthesis (In2O3/ZrO2) catalyst and a methanol-to-olefins (SAPO-34) catalyst. Under typical reaction conditions (e.g., 15 bar, 400 °C, and a space velocity of 12 L gcat−1 h−1), light olefins (ethylene and propylene) with a selectivity of 80–90% in hydrocarbons can be obtained with a CO2 conversion of ∼20%. To the best of our knowledge, this is the highest selectivity reported to date, which significantly surpasses the value obtained over conventional iron or cobalt CO2 Fischer–Tropsch synthesis catalysts (typically less than 50%). Moreover, our designed bifunctional catalyst shows good catalytic stability and can run for 50 h continuously without obvious activity decay. Our study provides an important contribution for CO2 conversion to value-added chemicals.
- This article is part of the themed collection: Catalysis Science & Technology 10th Anniversary Symposium


 Please wait while we load your content...
Please wait while we load your content...