Enhanced thermal stability of palladium oxidation catalysts using phosphate-modified alumina supports†
Abstract
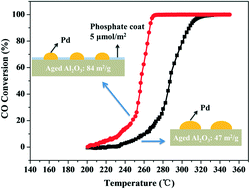
A group of phosphate-modified alumina materials with enhanced thermal stability was developed in this work. Using these oxides, supported Pd catalysts experienced much less deterioration after aging at 1050 °C for 10 h. The CO oxidation kinetic results showed that the phosphate additives did not change the intrinsic activity of the Pd catalytic centers and the larger population of sinter-resistant Pd species anchored on the phosphate-stabilized alumina was the direct reason behind this improved catalytic performance. Combining the analyses of NMR, IR, pyridine adsorption and DFT simulations, the phosphate adsorption and evolution behaviors on the alumina surface were explored at different phosphorus contents. The effective phosphate stabilizers prefer to coordinate with the surface coordinatively unsaturated –Al sites (CUS Al) and substitute the weakly adsorbed hydroxyls nearby. Such an interaction suppressed the phase transformation and grain growth of the alumina. Compared with the phosphate-free Pd catalyst, alumina with ∼5.0 μmol m−2 phosphorus additives is able to retain 1.9 times higher Pd dispersion after aging, resulting in much higher CO oxidation activity. Further increase of the phosphorus content induced the formation of long-chain polyphosphate and harmed the stability of Pd, bringing no more benefits to the performance of the catalysts.


 Please wait while we load your content...
Please wait while we load your content...