Further insight into the electrocatalytic water oxidation by macrocyclic nickel(ii) complexes: the influence of steric effect on catalytic activity†
Abstract
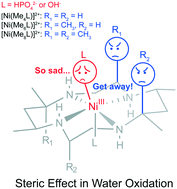
The development of efficient, robust and economical water oxidation catalysts (WOCs) remains a key challenge for water splitting. Herein, three macrocyclic nickel(II) complexes with four, six and eight methyl groups in the ligands have been utilized as homogeneous electrocatalysts for water oxidation in aqueous phosphate buffer at pH 7.0, in which the catalyst with eight methyl groups exhibits the highest catalytic activity, with a large current density of 1.0 mA cm−2 at 1.55 V vs. NHE (750 mV overpotential) in long-term electrolysis. The results of electrochemistry, UV-vis spectroelectrochemistry and DFT calculations suggest that the axially oriented methyl groups in the macrocyclic ligands with eight and six methyl groups can impose a steric effect on the axial position of the NiIII center, which not only results in higher NiIII/II oxidation potentials but also suppresses the axial coordination of phosphate anions with the NiIII center to achieve better catalytic performance. Such a steric effect in homogeneous WOCs has not been reported so far.


 Please wait while we load your content...
Please wait while we load your content...