Formation of C–C, C–S and C–N bonds catalysed by supported copper nanoparticles†
Abstract
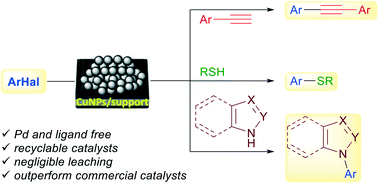
Transition-metal catalysed cross-coupling reactions are still dominated by palladium chemistry. Within the recent past, copper has gained ground against palladium by virtue of its cheaper price and equivalent function in certain reactions. Four catalysts consisting of copper nanoparticles on zeolite, titania, montmorillonite and activated carbon have been tested in three palladium- and ligand-free cross-coupling reactions to form carbon–carbon, carbon–sulfur and carbon–nitrogen bonds. CuNPs/zeolite has been found to be the best one in the Sonogashira reaction of aryl iodides and arylacetylenes, as well as in the coupling of aryl halides with aryl and alkyl thiols, being reusable in both cases. However, the arylation of nitrogen-containing heterocycles (imidazole, pyrazole, benzimidazole and indole) has been better accomplished with CuNPs/titania, albeit CuNPs/activated carbon showed better recycling properties. The catalytic activity of the nanostructured catalysts has been compared with that of twelve commercial copper catalysts, with the former outperforming the latter in the three types of reactions studied.


 Please wait while we load your content...
Please wait while we load your content...