Control of stereoselectivity of benzylic hydroxylation catalysed by wild-type cytochrome P450BM3 using decoy molecules†
Abstract
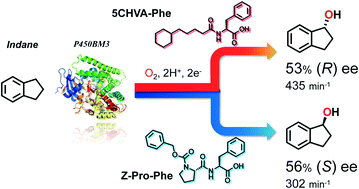
The hydroxylation of non-native substrates catalysed by wild-type P450BM3 is reported, wherein “decoy molecules”, i.e., native substrate mimics, controlled the stereoselectivity of hydroxylation reactions. We employed decoy molecules with diverse structures, resulting in either a significant improvement in enantioselectivity or clear inversion of stereoselectivity in the benzylic hydroxylation of alkylbenzenes and cycloalkylbenzenes. For example, supplementation of wild-type P450BM3 with 5-cyclohexylvaleric acid-L-phenylalanine (5CHVA-Phe) and Z-proline-L-phenylalanine yielded 53% (R) ee and 56% (S) ee for indane hydroxylation, respectively, although 16% (S) ee was still observed in the absence of any additives. Moreover, we performed a successful crystal structure analysis of 5CHVA-L-tryptophan-bound P450BM3 at 2.00 Å, which suggests that the changes in selectivity observed were caused by conformational changes in the enzyme induced by binding of the decoy molecules.


 Please wait while we load your content...
Please wait while we load your content...