Effect of O2, CO2 and N2O on Ni–Mo/Al2O3 catalyst oxygen mobility in n-butane activation and conversion to 1,3-butadiene†
Abstract
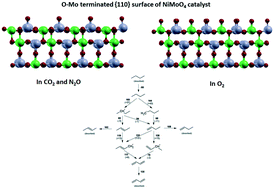
A commercial heterogeneous Ni–Mo/Al2O3 catalyst was tested for the oxidative dehydrogenation (ODH) reaction of n-butane with different oxidant species: O2, CO2 and N2O. The effect of the lattice oxygen mobility and storage in Ni–Mo/Al2O3 on catalytic conversion performance was investigated. Experiments indicated that a high O2-storage/release is beneficial for activity, however at the expense of selectivity. A significant amount of butadiene with no oxygenated compound products was formed upon using carbon dioxide and nitrous oxide, while O2 favoured the formation of cracked hydrocarbon chains and COx. The highest turnover yield to 1,3-butadiene was achieved at an oxidant-to-butane molar ratio of 2 : 1 at temperatures of 350 °C and 450 °C. With CO2, significant amounts of hydrogen and carbon monoxide have evolved due to a parallel reforming pathway. Partial nickel/molybdenum oxidation was also observed under CO2 and N2O atmospheres. TPR revealed the transformation of the high valence oxides into structurally distinct metal sub-oxides. In TPRO, three distinct peaks were visible and ascribed to surface oxygen sites and two framework positions. With N2O, these peaks shifted towards a lower temperature region, indicating better diffusional accessibility and easier bulk-to-surface migration. XRD revealed the presence of an α-NiMoO4 active phase, which was used in DFT modelling as a (110) plane. Theoretical ab initio calculations elucidated fundamentally different reactive chemical intermediates when using CO2/N2O or O2 as the oxidant. The former molecules promote Mo atom oxygen termination, while in an O2 environment, Ni is also oxygenated. Consequently, CO2 and N2O selectively dehydrogenate C4H10 through serial hydrogen abstraction: butane → butyl → 1-butene → 1-butene-3-nyl → butadiene. With O2, butane is firstly transformed into butanol and then to butanal, which are prone to subsequent C–C bond cleavage. The latter is mirrored in different mechanisms and rate-determining steps, which are essential for efficient butadiene monomer process productivity and the optimisation thereof.


 Please wait while we load your content...
Please wait while we load your content...