Water as a catalytic switch in the oxidation of aryl alcohols by polymer incarcerated rhodium nanoparticles†
Abstract
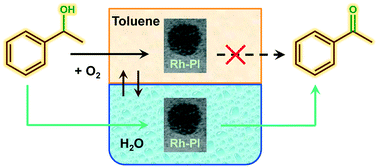
One of the major goals in the oxidation of organic substrates, and especially for alcohol oxidation, is the use of molecular oxygen as the oxidant under mild conditions. Here we report the synthesis and testing of Rh polymer incarcerated catalysts, using a metal so far not used for alcohol oxidation reactions, in which the catalytic activity towards aryl alcohol oxidation, for substrates like 1-phenylethanol and benzyl alcohol, is switched on by the addition of water as co-solvent in toluene. This is done by using air as oxidant at atmospheric pressure, in one of the mildest reaction conditions reported for this class of reaction. The promoting effect of water to higher conversions was observed also for rhodium over alumina supported catalysts, which were used as a benchmark allowing in all cases high conversion and selectivity to the ketone or the aldehyde within a short reaction time. The effect of water was explained as a medium capable to promote the oxidation of the alcohol to the ketone in a biphasic system assisted by phase transfer catalysis. This is particularly relevant for alcohols like 1-phenylethanol or benzyl alcohol that are not soluble in water at room temperature, and for which alternative oxidation routes are needed, as well as to switch on the catalytic activity of metal nanoparticles in a facile and green manner for the activation of molecular oxygen. Aliphatic alcohols like 1-octanol and 3-octanol were also tested, still showing Rh based catalysts as promising materials for this reaction if toluene only was used as solvent instead.
- This article is part of the themed collection: 2017 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...