The effect of surface chemistry on the performances of Pd-based catalysts supported on activated carbons†
Abstract
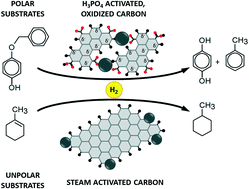
In this work we investigated in detail the effects of nitric acid on the surface chemistry of two carbons, activated by steam and by phosphoric acid, meant to identify the nature and the concentration of the oxidized surface species. To this aim, the oxidized carbons were characterized by means of a large number of complementary techniques, including micro-Raman spectroscopy, N2 physisorption, Boehm titration method, 13C solid state nuclear magnetic resonance, X-ray photoelectron spectroscopy, diffuse reflectance infrared and inelastic neutron scattering spectroscopy. Carboxylic and carboxylate groups are mainly formed, the latter stabilized by the extended conjugation of the π electrons and being more abundant on small and irregular graphitic platelets. We demonstrated that the presence of oxygen-containing groups acts against the palladium dispersion and causes the appearance of an appreciable induction time in hydrogenation reactions. The carbon with more oxygenated surface species (and in particular more carboxylate groups) must be chosen in the hydrogenation of polar substrates, while it is detrimental to the hydrogenation of nonpolar substrates.


 Please wait while we load your content...
Please wait while we load your content...