Switchable synthesis of furfurylamine and tetrahydrofurfurylamine from furfuryl alcohol over RANEY® nickel†
Abstract
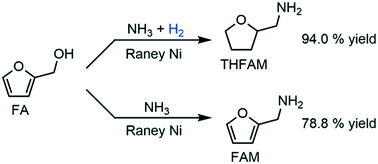
RANEY® Ni proved to be an effective heterogeneous catalyst for switchable reductive amination of furfuryl alcohol to tetrahydrofurfurylamine and furfurylamine with NH3 by simply adding or not adding 1.0 MPa H2 into the reaction bulk. After further optimization of the reaction conditions, we finally obtained 94.0% yield of tetrahydrofurfurylamine and 78.8% yield of furfurylamine with high selectivity. By extensively studying the catalytic pathways and mechanism of catalyst deactivation with XRD and XPS characterization, we have confirmed that an excess amount of H2 in the reaction bulk leads to the deep hydrogenation of the furan ring while an insufficient amount of H2 leads to the formation of Ni3N and the deactivation of the catalyst.


 Please wait while we load your content...
Please wait while we load your content...