Unravelling the radical transition during the carbon-catalyzed oxidation of cyclohexane by in situ electron paramagnetic resonance in the liquid phase†
Abstract
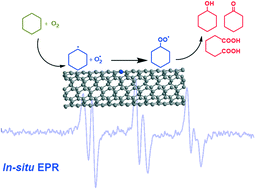
The selective oxidation of hydrocarbons is of great importance in the chemical industry. Nanocarbons have attracted intensive attention as metal-free catalysts in this field. Efforts have been made to reveal the crucial role of nanocarbons in the activation of C–H bonds and radical propagation in hydrocarbon oxidation, but it is still far from being understood. In this work, in situ Electron Paramagnetic Resonance (EPR) was conducted in the selective oxidation of cyclohexane (CyH) catalyzed by nitrogen-doped carbon nanotubes (N-CNTs) and multiwalled carbon nanotubes (MWCNTs) in the liquid phase. The interaction between the radicals and nanocarbons was disclosed. The unique role of N-CNTs as a catalyst in the selective oxidation of CyH for the production and propagation of C6H11˙ and O2˙ radicals was evidently confirmed. This work will provide new mechanistic insights into the oxidation of hydrocarbons in the liquid phase.


 Please wait while we load your content...
Please wait while we load your content...