In situ assembly of ultrafine Mn3O4 nanoparticles into MIL-101 for selective aerobic oxidation
Abstract
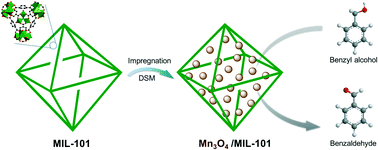
Nanosize metal oxides generally possess high catalytic activity, but they tend to agglomerate into larger particles during a reaction. Therefore, the preparation of nanosize metal oxides with high stability is a crucial challenge. A novel and facile approach involving impregnation followed by a double solvent method was developed to directly encapsulate ultrafine Mn3O4 nanoparticles (NPs) into the nanocages of metal–organic frameworks (MOFs). A series of MIL-101 encapsulated Mn3O4 NPs were prepared, in which the contents of Mn3O4 ranged from 3.2% to 33%. Ultrafine Mn3O4 NPs with a particle size of about 3 nm have been successfully embedded in the nanocages of MIL-101 with a uniform distribution. The MIL-101 encapsulated Mn3O4 NPs with a Mn3O4 content of 15% exhibits the highest conversion of benzyl alcohol (38.7%) and a >99% selectivity to benzaldehyde. Furthermore, after being repeatedly used 10 times, its catalytic activity is hardly changed. When the content of Mn3O4 NPs was further increased, the catalytic activity of the catalyst decreases, due to aggregated Mn3O4 particles with a large size which formed outside the MIL-101 matrix.


 Please wait while we load your content...
Please wait while we load your content...